Four batches of the life-saving drug that helps stop allergic reactions in their tracks have been recalled after they realized there was a defective part that affects how the medicine is administered.
Roughly 80,000 EpiPen 300 microgram adrenaline injection auto-injectors have been recalled worldwide by the company who makes them after they realized the problem.
The released a statement that says the "The failure of the auto-injector to activate may result in patients not receiving the required dose of adrenaline, resulting in the worsening of symptoms of anaphylaxis or anaphylactic reactions, which could be life-threatening."
Currently there are two confirmed reports of the auto-injectors failing to activate.
Affected Batches:
5FA665 - expiry April 2017
5FA6651 - expiry April 2017
5FA6652 - expiry April 2017
5FA6653 - expiry April 2017
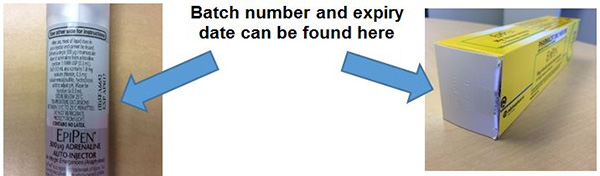
You can find your batch number and date on the box of the EpiPen or on the actual bottle itself.
They say that the EpiPen Jr 150 microgram and all other batches of the EpiPen 300 micrograms are unaffected.
If you have one of the affected batches, they can be replaced at no charge. They advise that until you can have them replaced that you CAN use them, they will just require more force to activate.
Share with everyone you know who has an EpiPen to keep them safe!

