If you're taking any kind of prescription medication for your high blood pressure, it's worth double-checking your medicine cabinet.
A pair of nationwide recalls, including tablets from different brand names and manufacturers, say that patients could face dangerous reactions or increase their cancer risk by taking unsafe or incorrect medication.
A Dangerous Mixup
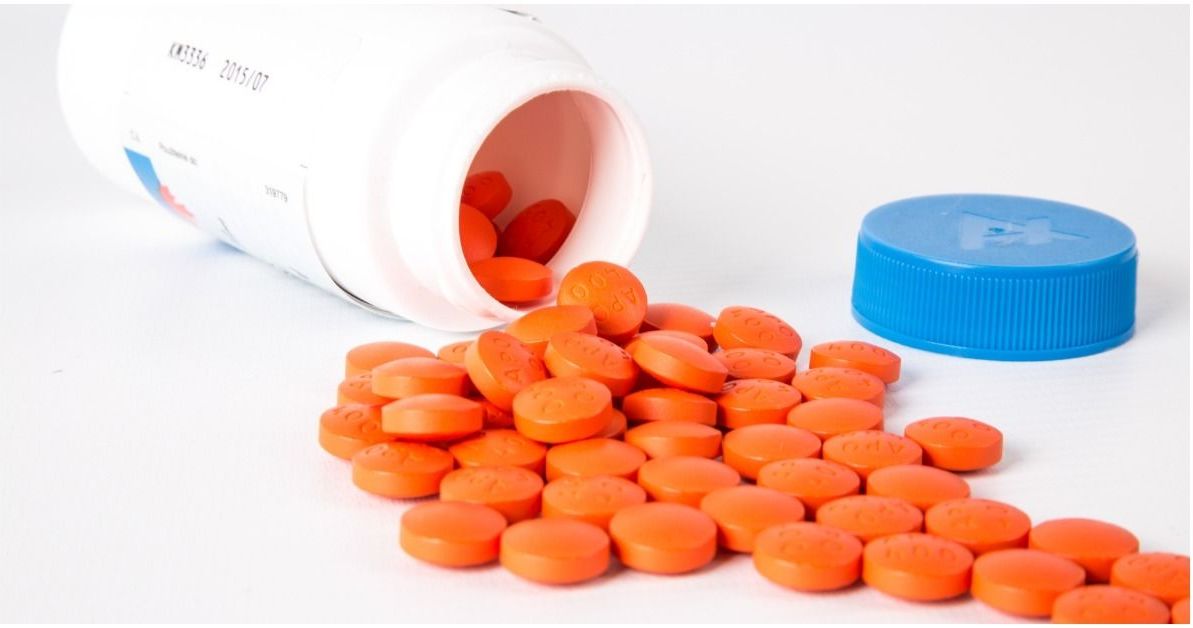
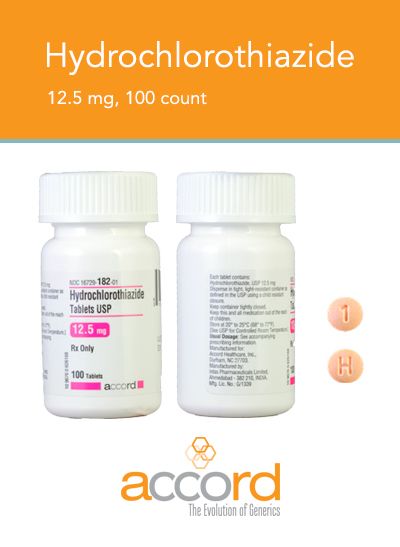
The latest recall is on bottles of hydrochlorothiazide 12.5 mg USP tablets (100 count).
A specific lot of the pill bottles is being voluntarily recalled by their manufacturer, Accord Healthcare, because they contain the wrong pills.
Instead of the blood pressure medication hydrochlorothiazide, the bottles were filled with 100 tablets of 25 mg USP spironolactone, which is used to treat congestive heart failure and cirrhosis.
The Food and Drug Administration (FDA) warns that patients taking the wrong medication could experience reactions ranging from "limited" to "life-threatening," because of an increase in potassium levels.
Accord says that only one lot of pills, PW05264, is involved in the recall.
They have also released a visual guide to identify the correct drugs.
The hydrochlorothiazide tablets are round, orange, and have "H" and "1" printed on opposite sides.
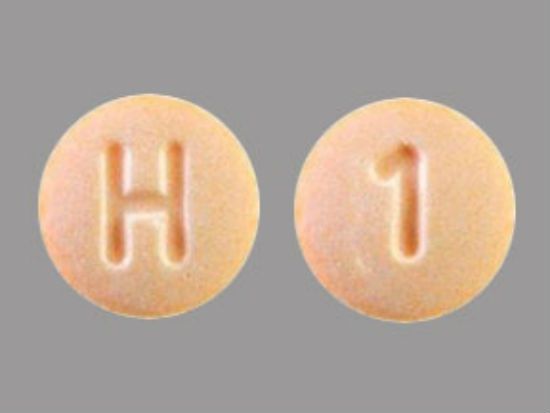
If you have Accord hydrochlorothiazide tablets that do not match that description, the FDA says to check with your pharmacist or healthcare provider.
The agency says so far no adverse reactions to the drugs have been reported, but you can report a reaction using their Medwatch form.
You can contact Accord Healthcare for more information by phone at 1-855-869-1081, fax: 1-817-868-5362 or e-mail at rxrecalls@inmar.com (Monday to Friday during business hours 8 a.m. to 5 p.m. EST.)
Another Recall Is Expanding
Last month, we told you that a number of generic blood pressure medications were being recalled because of an impurity that could increase a patient's cancer risk.
A number of drugs containing the ingredient valsartan and produced by the Chinese drug manufacturer Zhejiang Huahai Pharmaceuticals were found to contain NDMA.
NDMA is a chemical byproduct known to increase the risk of cancer in animals, and suspected of doing the same in humans.
The nationwide warning is part of a global recall of valsartan drugs produced by Zhejiang Huahai, which is still underway in more than 20 countries.
Not all drugs containing valsartan are being recalled, but this week the FDA expanded the list of drugs being recalled.
Check the label on your valsartan medication for one of these affected manufacturers:
- AvKare (Teva/Actavis) and (Hetero/Camber)
- A-S Medication Solutions LLC (Teva/Actavis & Prinston/Solco)
- Bryant Ranch Prepack Inc. (Teva/Actavis)
- Hetero Labs (labeled as Camber Pharmaceuticals Inc.)
- H J Harkins Co., Northwind Pharmaceuticals (Teva/Actavis)
- NuCare Pharmaceuticals Inc. (Prinston/Solco)
- Preferred Pharmaceuticals Inc. (Hetero/Camber)
- Prinston Pharmaceutical Inc. (labeled as Solco Healthcare LLC)
- Proficient Rx LP
- Remedy Repack (Prinston/Solco)
- Remedy Repack Inc. (Hetero/Camber)
- RemedyRepack Inc. (Torrent)
- Teva Pharmaceuticals (labeled as Major Pharmaceuticals)
- Teva Pharmaceuticals USA (labeled as Actavis)
- Torrent Pharmaceuticals Limited
Check here for a full list of drugs containing valsartan that are not being recalled.
If your bottle's label does not include the name of the manufacturer, contact your pharmacist.
You should also consult your doctor before stopping or changing your medications.
The FDA actually suggests patients who have tainted valsartan tablets should continue taking them until a safe replacement is provided.
If you experience a reaction from your medication, you can report it using the FDA's Medwatch form.